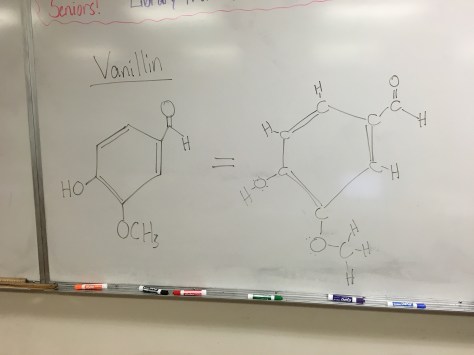
The final lesson of chapter 7 brings together student learning about how the olfactory system (the way we perceive smell) works. The Lesson 41 PowerPoint includes the key vocabulary concept of receptor site theory, where students learn the importance of molecule shape in determining recognition by receptor molecules involved in sensing smell. During class, we discussed how this concept is readily transferred across biology, extending beyond smell to areas like immunology. Students received the Lesson 41 Worksheet, with the option to research their favorite smell, determine whether the olfactory receptor for that smell is known, and to model their favorite smell molecule with MolView rather than construct a poster. Students also learned how to “read” an organic molecule structure traditionally used by chemists (see picture from the whiteboard below). For homework, students may complete any 4 of the textbook questions for this lesson.
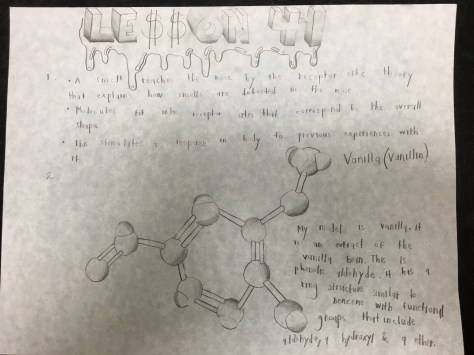
Student work:










You must be logged in to post a comment.