A huge thank you to Mr. Frank for overseeing a lab while I was away on a WABS professional development field trip to Renton Technical College. Students worked in small groups to complete the lab outlined in the Lesson 50 Worksheet. For future reference, students may also review the Lesson 50 PowerPoint slides that accompany the textbook reading. For homework, students were assigned textbook questions 5-7 from the end of Lesson 50 (or notes on the reading).
Category Archives: Chemistry
Weather: Weather Science
We launched Unit 3 with an overview of how to make sense of the various types of weather maps used to predict weather. The Lesson 49 PowerPoint, Lesson 49 Worksheet, and the Weather Variables handout are available for download. For more information about the jet stream, check out the short video below:
For weather forecast data, visit the University of Washington Atmospheric Sciences Virtual Map Room. Lesson 49 textbook questions 1, 2, and 6 were assigned as homework. To help connect the concepts of temperature, volume, and pressure, we also watched Kevin Delaney performing on Jimmy Fallon:
Molecular Structure and Properties: Amino Acids and Proteins
The final lesson of Unit 2 explores how amino acids connect to make proteins. The Lesson 48 PowerPoint includes the vocabulary terms of amino acid and protein. Lesson 48 connects with Lesson 47, as amino acids are chiral molecules. Notably, all of the 20 different amino acids in human proteins are “left-handed” (as opposed to the mirror-image “right-handed” isomers), meaning they all have the L conformation (L for laevus, Latin for “left”) rather than the D conformation (D for dexter, Latin for “right”). Students will work in pairs to complete the Lesson 48 Worksheet, learning about the properties of amino acids and how they bind together to form proteins. For more on the D and L convention, click on the picture below.
As noted previously, there are 20 different amino acids. All amino acids share the same base structure of a central carbon atom bound to a carboxylic acid (-COOH), an amino group (-NH2), and a hydrogen (H). The central carbon is also bound to an R group, with R indicating any one of the 20 different amino acid structures. The structures each have different physical properties. When individual amino acids link together, a polypeptide chain is formed (and a molecule of water is removed as each new amino acid is linked to the chain). The polypeptide chain, composed of a string of amino acids, folds into a particular shape determined by the interactions of all of the amino acids. The shape of a protein determines its function in the body. Mr. Anderson of Bozeman Science has a fantastic video explaining the nature of proteins:
For students looking for a good review of Central Dogma (DNA > RNA > Protein > Trait), the Crash Course Biology video DNA, Hot Pockets, & The Longest Word Ever is a good resource:
Finally, for students with access to a home computer, the Fold.It website will have you folding proteins in no time!
Molecular Structure and Properties: Mirror-Image Isomers
Welcome to second semester! We have a few new students joining our class, so we will briefly review the syllabus (with minor revisions), talk about class mechanics, and consider opportunities for fine-tuning how we use our new textbook this semester. Next, we will dive into Lesson 47 and investigate the concept of mirror-image isomers, also known as chiral compounds. We will begin with the Lesson 47 PowerPoint ChemCatalyst to help get students thinking about mirror images. We will then watch a short video about chirality (below):
Students will then receive the Lesson 47 Worksheet, working in pairs to model the compounds using the class set of molecular modeling kits. The worksheet concludes with students hypothesizing whether L-carvone will smell like D-carvone, and then testing their hypothesis. For homework, students were assigned textbook questions 2, 7, 8, and 9.
Want more? Check out the blog post Perhaps looking-glass milk isn’t good to drink for an overview of Lewis Carroll, looking-glass milk, and L- and D-carvone. Want more? Joanna Shawn Brigid O’Leary from Rice University published an even more extensive investigation of how Lewis Carroll (author of Alice in Wonderland and Through the Looking Glass) weaved biochemistry into his fiction. Her paper (available as a PDF), WHERE ‘THINGS GO THE OTHER WAY’: THE STEREOCHEMISTRY OF LEWIS CARROLL’S LOOKING-GLASS WORLD is well worth the read. Perhaps it will even inspire students to read the book before the movie is released in theaters on May 27!
Molecular Structure and Properties: Phase, Size, Polarity, and Smell
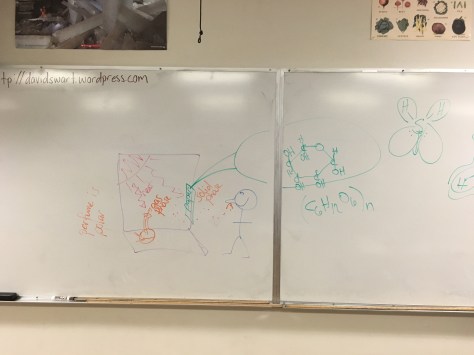
Chapter 8 concluded with the Lesson 46 PowerPoint and Lesson 46 Worksheet. Lesson 46 brought together the various concepts needed to understand how molecules with certain properties can be detected by our noses, with our brain recognizing those molecules as having a specific smell. The ChemCatalyst asks students to model why perfume molecules can be smelled from across a room, but paper cannot (both placed near a sunny window). We sketched out a drawing of the scenario and modeled the response on the white board (pictured below):
Molecular Structure and Properties: Polar Molecules and Smell
We continued our study of polarity, this time exploring how the polarity of molecules might impact our ability to smell the molecule. Through the Lesson 45 PowerPoint, students learned that polar molecules are more likely to be detected by the nose as something with a scent although there are still polar molecules (like water) that do not smell. Students worked in pairs to cut out the molecules in the molecules handout and used the molecules to complete the Lesson 45 Worksheet. Students who would like to explore the polarity of molecules further are encouraged to visit the University of Colorado’s PhET molecule polarity simulation (or just click below).
Molecular Structure and Properties: Electronegativity Scale
After learning about the concepts of electronegativity and polarity in yesterday’s lesson, students learned how scientist Linus Pauling assigned electronegativity values to individual atoms as a measure of how strongly an atom attracts electrons. The Lesson 44 PowerPoint includes a copy of the periodic table with electronegativity values for each element. It also explains the difference in electronegativity between covalent bonds (0.5 and less), polar covalent bonds (between 0.5-2.1), and ionic bonds (greater than 2.1). The Lesson 44 Worksheet provides students with the opportunity to calculate the electronegativity difference between two atoms in a molecule and to use that information to determine the type of bond that is present between the two atoms. For homework, students are assigned questions 1-9 (odds).
Molecular Structure and Properties: Electronegativity and Polarity
We began class with a Crash Course chemistry video about polar and non-polar molecules:
We then worked through the Lesson 43 PowerPoint and followed that with the Lesson 43 Worksheet and accompanying cartoon. One of the vocabulary terms, dipole, is introduced in the Crash Course video along with the concept of dipole moment. For more on dipole moment, check out the Khan Academy video below:
Dipole moment: Predicting the molecular dipole moment based on the molecular geometry.
https://www.khanacademy.org/embed_video?v=q3g3jsmCOEQ
For homework, students are assigned questions 2, 4, and 6.
Molecular Structure and Properties: Attractions between Molecules
Chapter 8 began with a lab designed to introduce students to the concept of polarity. Students filled burets with water, vinegar, and vegetable oil and observed the path of the liquids in the absence or presence of an electrically charged wand. The wand consisted of a long balloon rubbed vigorously on students’ hair to generate a static charge (build-up of electrons) on the balloon. Students then observed how the negatively-charged balloon “pulled” the stream of water toward the balloon (pictured below along with the lab set-up). Students worked through the Lesson 43 Worksheet while completing the lab. After the lab, we reviewed the key vocabulary and concepts presented in the Lesson 43 PowerPoint. For homework, students are assigned questions 4-6.
Molecular Structure and Properties: Chapter 7 Quiz
After the Chapter 7 Quiz, students were treated to the Crash Course: Orbitals video below which explains why electron orbitals, and thus molecules, are shaped the way they are:





You must be logged in to post a comment.