Monday, March 2, 2020: With the coronavirus (COVID-19) all over the news, we will take some time this week to better understand the science of the virus, learn about tools scientists use to study epidemiology, and investigate how treatments may eventually be developed. After providing students some time to formulate and share questions and concerns, we will watch a comprehensive overview prepared by Dr. Arnaud Fontanet of the Institut Pasteur on February 20, 2020:
During his highly informative presentation, Dr. Fontanet references several of the following websites:
- Coronavirus Global Dashboard (Johns Hopkins)
- Modeling Coronavirus Control (UToronto)
- ProMed: Real-time infection disease updates
- Virological.org: Novel 2019 Coronavirus Genome
- University of Minnesota COVID-19 Maps & Visuals
- Genomic epidemiology of novel coronavirus (HCoV-19)
- King County Public Health: Coronavirus Disease 2019 (COVID-19)
- CDC: Coronavirus Disease 2019 (COVID-19)
- CDC: Severe Acute Respiratory Syndrome (SARS)
- CDC: Middle East Respiratory Syndrome (MERS)
- C&EN Coronavirus Information
Coronavirus, SARS, MERS (and more) in the News
- 3/7/20 – Bloomberg: There Is a ‘Tipping Point’ Before Coronavirus Kills
- 3/7/20 – Seattle Times: Facts about novel coronavirus and how to prevent COVID-19
- 3/3/20 – STAT: Washington State risks seeing explosion in coronavirus cases without dramatic action, new analysis says
- 3/2/20 – KOMO News: Coronavirus in Washington state: 6 dead, 12 others infected
- 3/1/20 – NY Times: Coronavirus May Have Spread in U.S. for Weeks, Gene Sequencing Suggests
- 2/5/20 – PBS Frontline: Inside the Fight Against an Earlier, Deadly Coronavirus: SARS
- 1/27/20 – Slate: Do Surgical Masks Stop the Coronavirus?
- 5/5/15 – PBS Frontline: Outbreak (Ebola)
- 9/16/05 – HHMI: Learning How SARS Spikes Its Quarry
How About Treatment Options?
- 3/3/20 – Seattle PI – University of Washington researchers use online puzzle to crowdsource COVID-19 treatments
- Treatment Design: NIAID Immune Epitope Database
- 2/25/20 – National Geographic: Will warming spring temperatures slow the coronavirus outbreak?
- 2/25/20 – Time: COVID-19 Vaccine Shipped, and Drug Trials Start
- 4/16/19 – Frontiers in Microbiology: Structural Vaccinology for Viral Vaccine Design
- 9/10/14 – PBS NOVA: Vaccines – Calling the Shots (video)
Virus Outbreak Simulations
Get Proactive! The data is clear: the best way to control the spread of disease is through effective hand washing. Students looking to earn bonus credit are encouraged to bring in the following supplies (+5 bonus each, +20 bonus max):
- Hand Sanitizer
- Paper Towels
- Kleenex
- Cleaning Wipes
Earn an additional +5 bonus credit by taking the HHS Public Health Survey (Student Bathrooms).



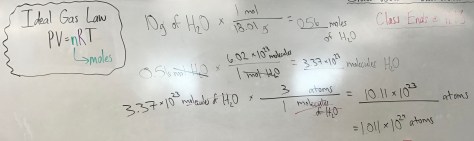



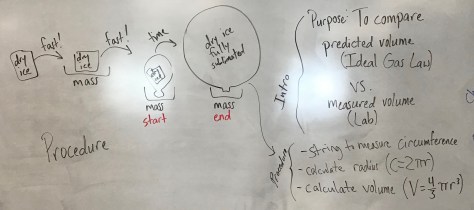









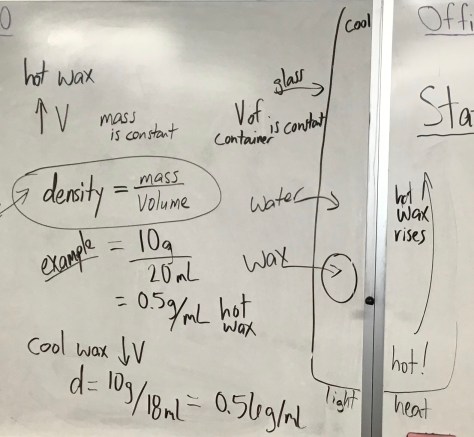






You must be logged in to post a comment.