We began with the Lesson 47 PowerPoint ChemCatalyst to help get students thinking about mirror images. We then watched a short video about chirality (below):
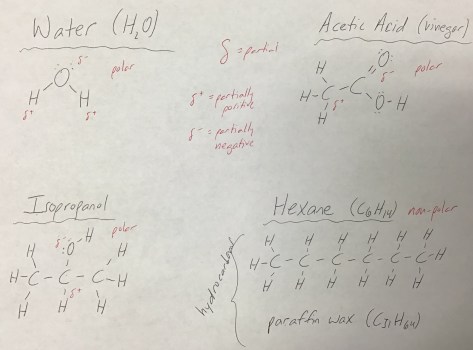
Students then received the Lesson 47 Worksheet, working in pairs to model the compounds using the class set of molecular modeling kits. The worksheet concluded with students hypothesizing whether L-carvone will smell like D-carvone, and then testing their hypothesis.
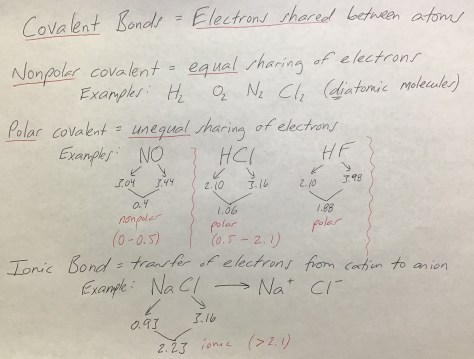
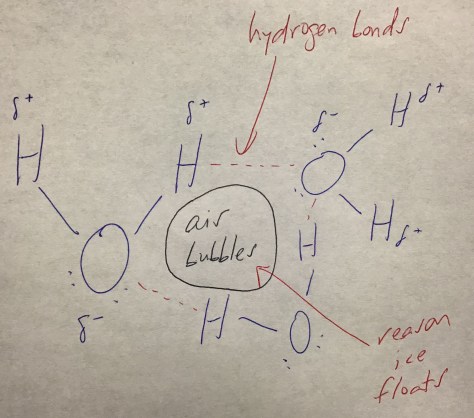
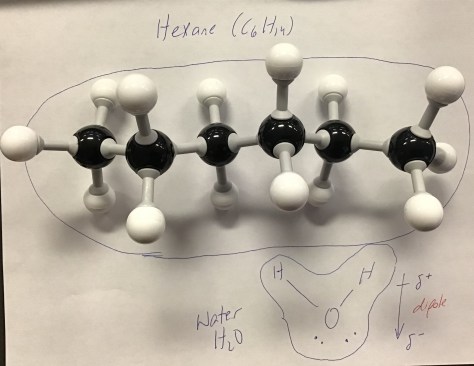
Class Notes


Keep Learning!
Want more? Check out the blog post Perhaps looking-glass milk isn’t good to drink for an overview of Lewis Carroll, looking-glass milk, and L- and D-carvone. Want more? Joanna Shawn Brigid O’Leary from Rice University published an even more extensive investigation of how Lewis Carroll (author of Alice in Wonderland and Through the Looking Glass) weaved biochemistry into his fiction. Her paper (available as a PDF), WHERE ‘THINGS GO THE OTHER WAY’: THE STEREOCHEMISTRY OF LEWIS CARROLL’S LOOKING-GLASS WORLD is well worth the read. Perhaps it will even inspire students to read the book before the movie is released in theaters on May 27!
Homework:
- Read Lesson 47 in the textbook. Login via hs.saplinglearning.com and enter your username and password:
- Username: wahps****s-####### (**** = first 4 letters of your last name and ####### = student number). Remember to include the dash between s and #.
- Password: S-####### (the S must be capitalized)
- Write notes for Lesson 47 on the Chapter 9 Notes handout.
- Work through the practice problems at the end of Lesson 47.
- Please ask questions about anything from Lesson 47 you do not yet fully understand.

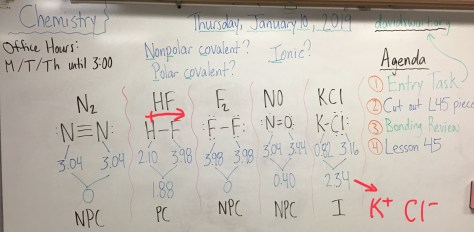
You must be logged in to post a comment.