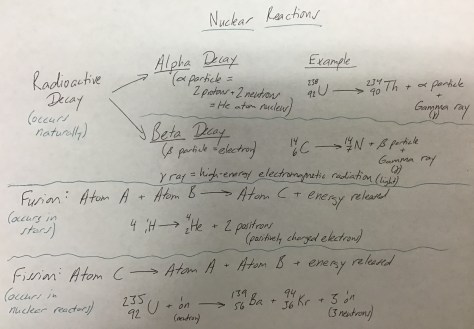
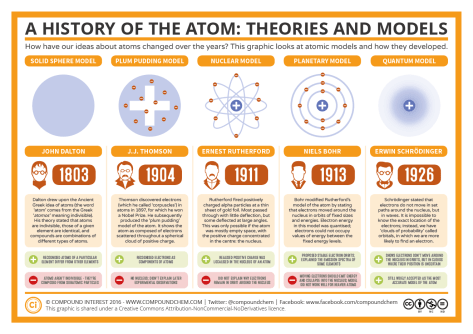
We continued our exploration of how the nucleus of an atom can change by launching into Lesson 15. The lesson addressed the question: What are nuclear reactions? We began with an illustration depicting the key vocabulary from the Lesson 15 PowerPoint in order to better understand the Nuclear Quest board game which is the hands-on learning for the day.
For the game, students gathered into teams of four, with each team receiving the board, the three sheets of nuclear quest cards, the two sheets of radiation cards and the game instructions. Students also received the Lesson 15 worksheet to help guide them through the key learnings in the game. Also, the game requires dice which are in limited supply, so visit Random.org and use the virtual dice roller!
Extend Your Learning!
- Keep learning through Khan Academy! There are a number of excellent lessons focusing on the life and death of stars (nuclear fusion reactions) as well as radioactive decay (nuclear fission reactions).
- Prefer learning through Crash Course? Watch the Nuclear Chemistry videos below:
- For more information on radioactive decay, visit the Science Education Resource Center’s Radioactive Decay web page at Carlton College.
- Investigate human efforts to harness and use nuclear reactions – we must understand history to avoid repeating it!
- The Atomic Bomb, Hiroshima, and Nagasaki (1945)
- Kyshtym (1957)
- Three Mile Island (1979)
- Chernobyl (1986)
- Fukushima (2011)
- Hanford, WA (1942 – present)
- France: A Nuclear Power Success Story
- NASA: Nuclear Thermal Propulsion
- Naval Base Kitsap / Bangor Submarine Base
- Nuclear Medicine
- The question of whether hydrogen is a metal or a nonmetal has come up in some classes in recent days. Settle the debate for yourself by reading the articles below:
Homework for this evening:
- Read Lesson 15 in the textbook. Login via hs.saplinglearning.com and enter your username and password:
- Username: wahps****s-####### (**** = first 4 letters of your last name and ####### = student number). Remember to include the dash between s and #.
- Password: S-####### (the S must be capitalized)
- Work through the homework problems at the end of Lesson 15 and then verify accuracy with the Lesson 15 Homework Answers.
- Write notes for Lesson 15 on the Chapter 03 Notes handout.
- Come to class tomorrow prepared to ask questions about anything of the homework problems from Lesson 15 you did not understand.



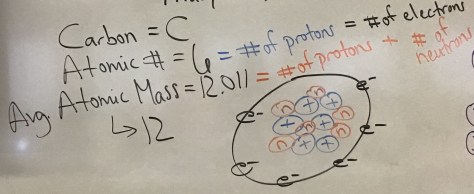
You must be logged in to post a comment.