With the Unit 3 exam scheduled for tomorrow, we took class time today to review key concepts for the exam. We focused much of the review on the concept of moles, with students having access to a set of practice problems and the answer key. We worked through most of the problems in class and the work from the white board is pictured below:
Category Archives: Chemistry
Weather: Extreme Physical Change
For the final lesson of Chapter 12 (and Unit 3!), students applied their understanding of temperature and pressure to the extreme weather example of hurricanes. We began class with a video describing how hurricanes form:
After the video, we briefly reviewed the lesson objectives in the Lesson 67 PowerPoint. Students then received a packet containing the Lesson 67 Worksheet, a handout showing the anatomy of a hurricane, a list of extreme weather occurrences from 2005, and a graph of global temperature changes from 1880-2012. For homework, students were assigned textbook questions 1 and 4.
Weather: Humidity, Condensation
For Lesson 66, students learned the definition of humidity on slide 5 of the Lesson 66 PowerPoint. Students then broke into groups and conducted the lab outlined in the Lesson 66 Worksheet. The worksheet packet also included a copy of the Relative Humidity Handout to use for calculations. For homework, students may work through any 4 problems from the textbook (or take notes on the reading – always an option!). Students were also reminded that we will have the Chapter 12 quiz next Tuesday and the Unit 3 exam next Thursday.
Weather: Ideal Gas Law
After several lessons learning about the component parts and relationships mathematically connecting pressure, volume, temperature, and number of particles, the Ideal Gas Law was revealed. We worked through the Lesson 64 worksheet and then watched a Crash Course video on the Ideal Gas Law:
After the video, we worked through Lesson 65 textbook problems 3 and 5. The notes from the white board are shown below. The Lesson 65 PowerPoint and Lesson 65 Worksheet are available for students who would like to see them. We did not use either today in class, and the Lesson 65 Worksheet was not assigned.
Weather: The Mole and Avogadro’s Law
For Lesson 64, students learned about standard temperature and pressure (STP), the Mole, and Avogadro’s Number. We worked through slides 5-7 of the Lesson 64 PowerPoint and then watched the Crash Course video below:
Students then received the Lesson 64 Worksheet. Interested in how scientists calculated the number of particles in one Mole? Read about it at Wired Magazine.
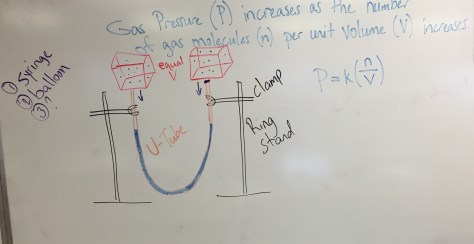
Weather: Pressure and Number Density
Happy Pi Day! We began class with a SciShow video about Pi (below) and students received an additional 20 minutes to finish the Chapter 11 quiz from Friday. We then moved into Lesson 63, the first lesson of Chapter 12. Students received copies of the Lesson 63 Worksheet, a companion handout with information connecting air pressure and temperature, and a calendar detailing lessons, assignments, and tests through the end of March. Students are also welcome to review the Lesson 63 PowerPoint. A picture of the white board with notes about the lab are also provided below.
Weather: High and Low Air Pressure
For the final lesson of Chapter 11, students conducted the”cloud in a bottle” lab. They added warm water to two-liter bottles and then filled the bottles with smoke. By squeezing and releasing the bottles, students observed cloud formation. They repeated the experiment with bottles filled with cold water and without water and made observations on the Lesson 62 Worksheet. The Lesson 62 PowerPoint is available here as a resource. In place of homework, students were encouraged to prepare a page of notes to use for the Chapter 11 Quiz tomorrow. Pictures of the whiteboard, including a list of key notes to take for the quiz tomorrow and some thinking about warm and cold front interactions.
Weather: Combined Gas Law
For Lesson 61, students were introduced to the Combined Gas Law, k=PV/T. We worked through the Lesson 61 PowerPoint and then students received the Lesson 61 Worksheet. During class, we also watched a portion of a video showing the effect of atmospheric pressure on balloon volume. For homework, students were assigned three textbook problems of their choosing.
Weather: Molecular View of Pressure
We began class with the Meters, Liters, and Grams video:
After the video, we briefly reviewed the Metric System:

We worked through a few practice problems from Appendix A in the textbook (page A-0) which integrate the metric system and dimensional analysis. After the
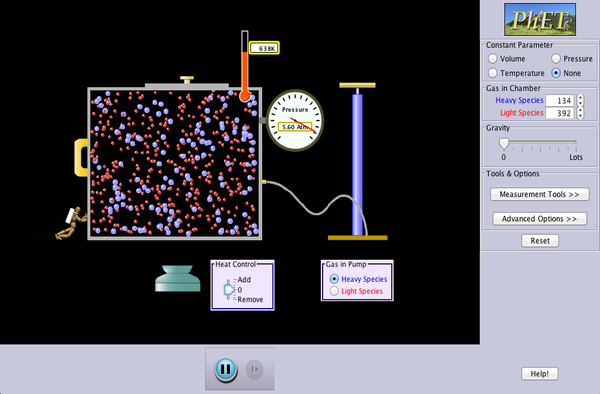
We then transitioned to Lesson 60, providing students the opportunity to make connections between Charles’s Law, Boyle’s Law, and Gay-Lussac’s Law. Although we did not review it in class, the Lesson 60 PowerPoint is available for students to review. The Lesson 60 Worksheet called for students to use a University of Colorado PhET simulation. Because our Chromebooks are unable to run Java, students instead observed a teacher-led demonstration of the simulation software. For homework, students were assigned textbook problems 3-9 (odds). Students with access to a Windows-based computer are encouraged to try the simulation (embedded below):
Weather: Gay-Lussac’s Law
For lesson 59, we learned about Gay-Lussac’s Law (P=kT), the third gas law needed to connect pressure, volume, and temperature. Gay-Lussac’s Law helps explain the egg-in-a-bottle trick, where boiled water displaces the air inside a bottle, and as the water condenses, an egg placed over the bottle will be pulled inside because of the change in pressure inside the bottle. The Lesson 59 worksheet and Lesson 59 PowerPoint are available for download.













You must be logged in to post a comment.