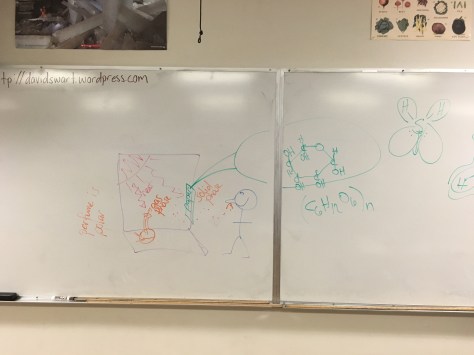
Chapter 8 concluded with the Lesson 46 PowerPoint and Lesson 46 Worksheet. Lesson 46 brought together the various concepts needed to understand how molecules with certain properties can be detected by our noses, with our brain recognizing those molecules as having a specific smell. The ChemCatalyst asks students to model why perfume molecules can be smelled from across a room, but paper cannot (both placed near a sunny window). We sketched out a drawing of the scenario and modeled the response on the white board (pictured below):




You must be logged in to post a comment.