We began class with the Meters, Liters, and Grams video:
After the video, we briefly reviewed the Metric System:

We worked through a few practice problems from Appendix A in the textbook (page A-0) which integrate the metric system and dimensional analysis. After the
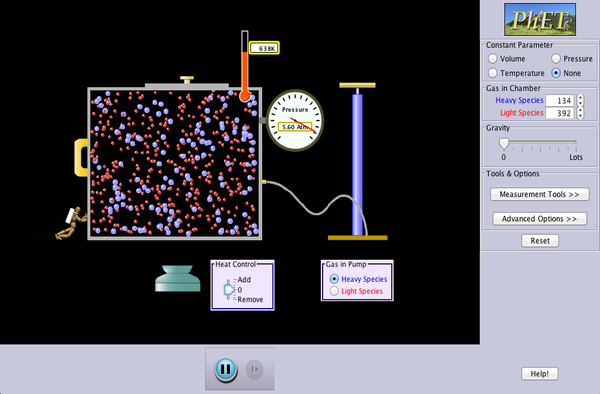
We then transitioned to Lesson 60, providing students the opportunity to make connections between Charles’s Law, Boyle’s Law, and Gay-Lussac’s Law. Although we did not review it in class, the Lesson 60 PowerPoint is available for students to review. The Lesson 60 Worksheet called for students to use a University of Colorado PhET simulation. Because our Chromebooks are unable to run Java, students instead observed a teacher-led demonstration of the simulation software. For homework, students were assigned textbook problems 3-9 (odds). Students with access to a Windows-based computer are encouraged to try the simulation (embedded below):



You must be logged in to post a comment.