This week’s bonus credit opportunity is called, “Movie Time!” To earn +10 bonus credit in the assignment category, you must share with me the title, a brief summary (must be your own writing!), and an explanation of why this movie is your all-time favorite science-related movie. Click here for the bonus credit Google Form. I will compile submissions into an anonymous list of movies and summaries below so we all have a resource to come back to this summer.
All posts by David Swart
Weeks 37 – Human Evolution
Welcome to Week 37! This week, we will step back in time and learn about the human branch of the evolutionary tree.
Last week was the latest in a long string of really tough weeks for our country. Rather than try making a light-hearted video introduction, I am simply asking you to visit the Future Voter registration page on the Washington Secretary of State website. You can register to vote as early as age 16 so you can then exercise your right to vote as soon as you turn 18. For many of you, turning 16 is still a year or two away. Remember, you also cast your vote every time you spend money. You have a choice about where to spend money, so make sure the money you spend is going to people, businesses, and causes that are worthy of your support. Finally, commit to lifelong learning. Your high school education is just the beginning. Please make the most of it. Education opens doors you may not even realize are there. Be brave, open all the doors, and keep them open for all who come behind you. Let’s get to it.
- Week 37 Attendance Check-In (required by 10am 6/5)
- The Origin of Humans (two Google Form assignments)
- The Science of Evolution (optional learning extension – no assigned work)
You did it! Just to make sure, here’s a checklist of items you must complete this week by Sunday, June 7 at 11:59pm:
- Week 37 Attendance Check-In (school district requirement)
- Human Origins Google Form (worth +15 assignment points)
- Australian Museum Article Summary Google Form (worth +30 assignment points)
Remember, you can email me any time. Office hours for Science are Tuesdays from 11am-12pm and Thursdays from 1pm-2pm. Check your student Gmail for Zoom instructions.
Don’t forget to complete the Week 37 Bonus Credit Opportunity! For a complete list of all of the bonus credit opportunities, bonus assignments, and bonus lab reports offered during distance learning, click here.
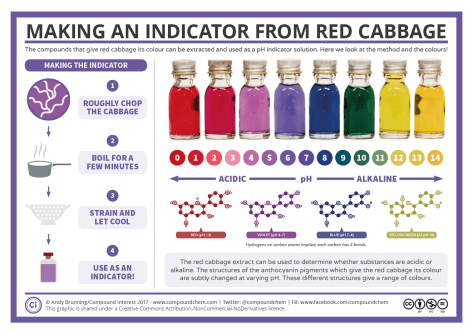
Week 37 – pH Indicators
To determine whether a solution is an acid, neutral, or a base, we need a tool. In the lab, we can use a pH probe to obtain a quantitative pH value (an actual number). Back in the day, we used pH strips to estimate the pH of different solutions. Some strips are more sensitive than others, but the common theme is the strips rely on the user matching the color of the strip to a color chart which then estimates the pH.
At home (or in high school classrooms with limited funding) we can create a colorimetric indicator using cabbage! Colorimetric pH indicators provide us with a semi-quantitative measurement of pH. By comparing the color of the indicator to a scale showing the color at a known pH, we can estimate the pH visually. Color alone would be a qualitative data point (a description) while matching the color to a number provides us with a quantitative data point (an actual number).
To be fair, scientists use laboratory-grade colorimetric indicators in the lab all the time, and then use machines called spectrophotometers to quantitatively determine the optical density of the light passing through…remember the ELISA post from last week?
If you watched the Week 36 Intro video, you will soon realize that it also introduced you to this part of our lesson. Prepare to be dazzled by the wizardry that is red cabbage juice indicator:
What is the actual chemistry behind red cabbage juice indicator? Click the picture below and find out:
You now have what you need to complete the pH Analysis Gizmo. The Gizmo was sent as a PDF attachment on Monday morning at around 8:00 am to the Week 37 – Chemistry Lesson email.
Anticipated answers to the question, “How do I turn in the Gizmo?”
- If you have access to a printer, print the Gizmo and then:
- Scan and email your completed work to Mr. Swart
- Send pictures of your completed work Mr. Swart
- Insert pictures of your completed work into a Google Doc and share with Mr. Swart.
- If you do not have access to a printer:
- Write answers on a piece of paper and then see above.
- Write answers in a Google Doc and then see above.
- Add comments to the PDF and share with Mr. Swart
- This is 2020 – get creative!
Extend your learning! For more on acid-base indicators, read Lesson 117 in the online textbook. Note: this is not an assignment and you are not required to turn in any work related to lesson 117.
Return to Week 37 – Acids and Bases and continue working.
Week 36 – What is a Phylogenetic Tree?
Our learning in Week 32 focused on phylogenetic trees. If you completed the work for that lesson, review your work to get re-acquainted with phylogenetic trees.
You are also encouraged to watch the Bozeman Science video below. Mr. Anderson explains how to construct a phylogenetic tree (also called a cladogram):
Looking for more background knowledge? Visit the UC Berkeley Understanding Evolution site and work through the Phylogenetics Tutorial.
Need more help? Click here for a Phylogenetic Tree Project Example.
Return to Week 36 – Phylogenetic Tree Project and continue working.
Week 36 – Solution Concentration
Welcome to Week 36! This week will tackle the concept of Solution Concentration. Many of the experiments we conduct in chemistry use chemicals in aqueous form. By the end of this lesson, you will know how to prepare chemical solutions of a specific molarity. Let’s go!
Checklist for the week:
- Week 36 Attendance Check-In (required by 10am 5/29)
- Molarity (Google Form assignment)
- Dilution (Google Form assignment)
- ELISA (optional virtual lab with assignment)
You did it! Just to make sure, here’s a checklist of items you must complete this week by Sunday, May 31 at 11:59pm:
- Weekly Attendance Check-In (school district requirement)
- Molarity (worth +10 assignment points)
- Dilution (worth +10 assignment points)
- Optional Lab Assignment (worth +30 bonus lab report points)
Remember, you can email me any time. Office hours for Science are Tuesdays from 11am-12pm and Thursdays from 1pm-2pm. Check your student Gmail for Zoom instructions.
Don’t forget to complete the Week 36 Bonus Credit Opportunity!
Week 36 – ELISA
As a biotech researcher for many years, one of lab techniques I used quite often was the Enzyme-Linked Immunosorbent Assay (ELISA for short). For many years, my research focused on understanding the activities of molecules of the immune system called interleukins (part of the cytokine family of molecules). Some of my work included focusing on understanding the biology of a interleukin called interleukin-17A (IL-17A). I maintained a line of mouse fibroblast cells (NIH-3T3 cells) which had been shown would respond to IL-17A and produce IL-6 (another interleukin), measurable by ELISA (click here for the ELISA procedure). If we stimulated the cells with IL-17A along with small amounts of other molecules (TNFα or IL-1β), we would see absolutely massive amounts of IL-6 released – well beyond what would be produced by the cells in response to any one of those molecules alone. At the time, we used this concept to screen blocking antibodies against the IL-17A receptor (IL-17RA) which were then used in a variety of mouse models of different diseases. Fast forward to today: scientists are accumulating data that the cytokine storm observed in some of the sickest COVID-19 patients may actually the result of those patients releasing too much IL-6, perhaps as a result of the activity of IL-17A released by the body as part of the defense against the virus. In fact, there is a clinical trial underway for an antibody to IL-6 called tocilizumab.
With all that as background, it’s time to focus our learning around the ELISA. For starters, if you are using an ELISA kit (like the IL-6 kit I linked to above), most of your assay reagents come packaged up in a tidy little box, and most of the reagents arrive as solids. To use the reagents, you need to add specific volumes of specific solutions (solution concentration!). After preparing the ELISA plates to receive samples, the protein standard must be diluted to the proper starting concentration. Then the protein standard is serially diluted to generate a standard curve. The samples are also often serially diluted. When the assay is complete, if all goes according to plan, you can use the standard curve to determine the concentration of protein in your samples.
Your turn! While not required, you are highly encouraged to work through the HHMI Biointeractive Immunology Virtual Lab. To guide your learning, complete the Immunology Virtual Lab Worksheet and earn +30 bonus points in the lab report category of your semester grade.
Return to Week 36 – Solution Concentration and continue working.
Week 36 – Basic Plant Biology
This year, we’ve learned a bit about plants – here are some highlights:
- The chemical equation for photosynthesis (Week 4) tells us that plants use sunlight energy, carbon dioxide, and water to produce molecules of glucose and oxygen.
- Plants have a cell wall and remain turgid in a hypotonic solution (Week 8).
- Plants are producers (Week 12), meaning they are the bridge between the sun (energy) and consumers (animals that need energy).
- Plants are central to the biogeochemical cycle (Week 14 and Week 15).
Now that we all agree that plants are really important and we wish we could have learned more about them, let’s make the most of our limited time and invest a few minutes learning more about plants:
Seeds are amazing! They are a little packet of starter nutrients and information (DNA) – and from a seed you can grow an entire plant which then produces more seeds! Let’s appreciate the wonder of seeds by watching the video below showing seed germination:
Admit it – you really want to grow some plants now, right? If you have access to some seeds and some soil, get to it! If you have access to some scissors and some mint plants, take a cutting, place the cutting in water, wait a week, and your new mint plant will sprout roots and be ready to plant as a new mint plant! Click here to meet Mr. Swart’s new mint plant starts. If not, no worries – I’ve got you covered. Back on April 25, the Swart Family Vegetable Garden was planted with seeds of 40 different types of vegetables. Our work this week will involve a journey through the garden…
Return to Week 36 – Inferring with Evidence and continue working.
Week 36 – Inferring with Evidence
Welcome to Week 36! With the school year winding down, it’s time to get outside and explore nature through the lens of evolution. Our work this week is to use evidence to infer relationships among a variety of vegetable plants commonly found in the garden. Let’s get to it!
- Week 36 Attendance Check-In (required by 10am 5/29)
- Basic Plant Biology
- Show Me the Veggies!
You did it! Just to make sure, here’s a checklist of items you must complete this week by Sunday, May 31 at 11:59pm:
- Week 36 Attendance Check-In (school district requirement)
- Phylogenetic Tree Project or Dichotomous Key Project (Google Doc, each worth +40 project points – pick one for full credit or complete both for +40 bonus credit)
Remember, you can email me any time. Office hours for Science are Tuesdays from 11am-12pm and Thursdays from 1pm-2pm. Check your student Gmail for Zoom instructions.
Don’t forget to complete the Week 36 Bonus Credit Opportunity!
Week 36 – Dilution
Often when you are working with chemicals in the lab, the chemicals are already in solution. For example, imagine you need to use some sodium hydroxide, NaOH, in a chemical reaction. You have a 1.0 L bottle of 1.0 M NaOH on the shelf, but your reaction calls for a 0.25 M solution. What to do? Prepare a dilution by adding solvent (in this case, water) to the solution to lower the concentration of the solute (in this case, NaOH).
For starters, we need to know the volume of 0.25 M NaOH that we need. Let’s say we need to end up with 1.0 L of the 0.25 M NaOH. Now we can figure this out. We know that M = mol/L. For our 0.25 M solution, M = 0.25 and L = 1.0. Rearranging the equation to solve for moles and we get mol = M x L = 0.25 x 1.0 = 0.25 mol. Therefore, we need to end up with 0.25 mol of NaOH in 1.0 L of solution.
Our stock solution of NaOH has a molarity of 1.0 M, or 1.0 mol / L. We need 0.25 moles of NaOH. To figure out the volume of stock solution we need to obtain 0.25 moles of NaOH, we can set up a proportion: 1.0 mol / 1.0 L = 0.25 mol / x. Solving for x, we need 0.25 L of the stock solution.
Finally, now that we know the volume of 1.0 M NaOH stock solution needed to add to prepare our 0.25 M NaOH solution (0.25 L), we need to calculate how much water to add to make the 0.25 M NaOH solution. We need a total volume of 1.0 L, and 0.25 L is going to come from the 1.0 M NaOH stock solution. Therefore, we need 1.0 L – 0.25 L = 0.75 L of water. To prepare the 1.0 L 0.25 M NaOH solution, we need to add 0.75 L of water to our flask, then add 0.25 L of the 1.0 M NaOH stock solution.
Now that you have seen the math and read the reasoning behind it in painstaking detail, let’s try a practice problem.
Question: How would you prepare 2.0 L of a 0.5 M aqueous solution of CuCl2 from a stock solution of 3.0 M CuCl2?
Answer: 0.33 L of the 3.0 M stock solution + 1.67 L of water. Why? A 3.0 M CuCl2 solution has 3.0 mol of CuCl2 per liter of solution. We want to prepare 2.0 L of a 0.5 M solution, so solving M = mol/L for mol, mol = M x L, so we need 2.0 x 0.5 = 1.0 mol of CuCl2 in a total volume of 2.0 L. Our stock solution is 3.0 mol/L so 1.0 mol = 0.33 L. Therefore, we need to add 0.33 L of the stock solution to 1.67 L of water.
One more question: Vinegar is commonly sold as a 5% acetic acid solution (the other 95% is water). A 100% acetic acid solution is called glacial acetic acid: glacial because the freezing point is just a few degrees below normal room temperature, so the acetic acid appears like a partially frozen glacier.
The molarity of glacial acetic acid is 17.4 M. How would you prepare 0.5 L of 1.0 M acetic acid?
Answer: You want to prepare 0.5 L of a 1.0 M acetic acid solution. First, calculate how many moles you need: mol = M x L so 0.5 x 1.0 = 0.5 mol of acetic acid. Next, the stock solution of glacial acetic acid has a molarity of 17.4 M, or 17.4 mol/L. To determine the volume of glacial acetic acid needed to obtain 0.5 mol: 0.5 mol x (1 L / 17.4 mol) = 0.029 L. Always add acid to water, so first add 0.471 L of water (0.5 L – 0.029 L) to the flask and then add 0.029 L of glacial acetic acid.
Time to show what you know! Complete the Week 36 – Dilution Google Form assignment and then return to Week 36 – Solution Concentration and continue working.
Week 36 – Show Me the Veggies!
The pictures below are of 25 different garden vegetables that were only seeds three weeks ago. Some plants clearly grow faster than others. In fact, quite a few seeds have yet to germinate, so this project isn’t quite as big as it could have been! What project? I’m glad you asked! This week, you have a choice. For either project, you will observe the plants carefully, writing down your observations for each plant in a Google Doc. Using your observations as evidence, you will either construct a phylogenetic tree or a dichotomous key. Both are worth 40 project points each. You must do one, you may do both. Doing both projects will earn you 40 bonus project points. Select the project you would like to complete and click on the link below for details.

























Return to Week 36 – Inferring with Evidence and continue working.

You must be logged in to post a comment.